- Among the Foundation’s multi-faceted missionsare research, establishing standards of practice and training, promote education of physicians, educate the public about the specialty, establish exchange programs with physicians of other countries, including training physicians in third world nations in plastic surgery techniques as well as treating citizens of those countries who do not have access to this kind of care.
- In 1946, Dr. Aufricht arranged with publisher Williams & Wilkins to publish the “Journal of Plastic and Reconstructive Surgery17.” The journal became the official journal of the ASPS, and the forum for publishing research, knowledge and discoveries in the plastic surgery specialties.
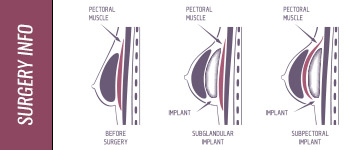
- The 1950s was a decade of discoveries and new techniques. Many new techniques were discovered and tested Korean war-zone hospitals. Public awareness of an expanding scope of procedures became more commonplace during the 1960s. Silicone was first used to treat skin imperfections, but, in 1962, one innovative physician, Drs. Thomas Cronin and Frank Gerow18 of Houston, Texas unveiled his use of silicone in a breast implant.
- The plastic surgery specialty reached a milestone in 1969 when President Nixon appointed Hal. B. Jennings, M.D. Surgeon General of the United States. Dr. Jennings was the first plastic surgeon to serve in this prominent position.
- During the 1970s ASPS members began changing some fundamental scopes of medical practice. Joseph Murray, M.D. of Boston, Massachusetts accomplished the first successful kidney transplant and flame-retardant children’s clothing was introduced as a lifesaving development of George Crikelair, M.D.19 of Florida.
- During the last half of this decade the Federal Trade Commission (FTC) took actions that would impact the entire medical community, requiring the ASPS to turn over some of their records to the FTC. After the FTC forced the American Medical Association to adopt what it had previously considered an unethical practice, that of advertising, the ASPS fought back in the name of quality medical care.
- The FTC saw board exams and certifications as an anti-competitive practice, although these are, in fact, assurances that patients are being treated by qualified physicians capable of offering the highest quality services. The ASPS reached another milestone when the FTC backed down, allowing this professional organization to determine how best to serve their patient community.
- During the 1980s, the ASPS saw an advantage in conceding on the idea of advertising, and undertook a more businesslike approach, which worked in its favor. They began producing information brochures and information products that educated patients on procedures, outcomes, and alternatives to help them make informed decisions about the treatments available to them and the growing number of plastic and cosmetic surgical procedures.
- Change accelerated during the 1990s.With over 5,000 active, certified plastic surgeons in the U.S. the Internet now provided a way for this widespread community to communicate easily with one another, as well as with many at one time. Online memberships to “Plastic Surgeons Online” provided an outlet for professionals to share questions and answers about cases, and hold internet conferences. The ASPS’ web site, http://www.plasticsurgery.org, offers the largest library of plastic surgery procedural information.
- Surveys indicated that the general public thought of plastic surgery as being only cosmetic surgery, posing a public relations problem for this specialty practice. The previous society name of American Society of Plastic and Reconstructive Surgeons implied that there were dual professions of Plastic Surgeons and Reconstructive Surgeons, thus giving rise to the understanding that Plastic Surgery and Cosmetic Surgery were synonyms and interchangeable.
- This misnomer worsened when multiple unrelated events created a rush of negative publicity for silicone breast implants. Breast implants had been designated class II medical devices in the 1970s. In 1988, the FDA reclassified them as class III devices, which required manufacturer safety and effectiveness studies. In 1989 a manufacturer withdrew polyurethane foam-covered implants from the market because a study raised FDA concern. Nearly two years later, in December 1990, Connie Chung’s infamous news story about the horrors ofsilicone implants sparked a number of lawsuits, and with them, government intervention.
- The FDA issued a moratorium on breast implants in January 1992, and by April, it had restricted implants to those women undergoing reconstruction under clinical studies. Silicone breast implants were no longer available. In September 1993 the manufacturers of silicone breast implants settled a class action suit for over $4 billion. But this suit turned out to have an interesting twist, as you’ll see.
⤛ Cosmetic Surgeons, Procedure Information, & Cosmetic Surgery News ⤜
Welcome to The Cosmetic Surgery Directory, the authoritative resource for people interested in plastic surgery!
This consumer-friendly website can be used as a central resource to answer your questions about plastic surgery and to locate a qualified cosmetic surgeon near you.
Here you can find detailed information on procedures or search an extensive directory of cosmetic surgery articles.
Contact us today for more help with your cosmetic surgery needs.
Top Story

FDA Proposes New Warnings on Breast Implants
The U.S. Food and Drug Administration (FDA) says that it has heard from women who feel they are not fully informed about the risks of breast implants and need more information in order to have … [Read More...]